Basics of Electrochemistry
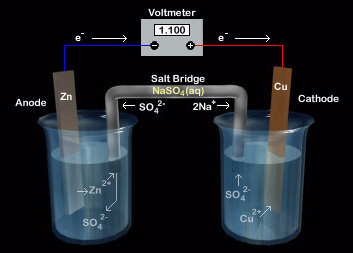
Basic electro-chemical processes such as using redox reactions to create a flow of electrons are the basis for how batteries work. Most batteries or cells are based off of the galvanic cell. Good examples of batteries based off of galvanic cells are dry cell batteries commonly used in flashlights and transistor radios; lead storage batteries which are your car batteries; and lithium-ion batteries normally found in cell phones, digital cameras, laptops, and electric vehicles. Galvanic cells contain cathodes and anodes with some form of an electron salt bridge. The cathode is negatively charged, where reduction occurs, and where electrons are gained. The opposite end of the spectrum on the battery is the anode which is positively charged and where electrons are lost. It should be noted that this is when the cell is operating. Salt bridges help facilitate the longevity of the battery and complete the circuit for the flow of electrons. Without the salt bridge electrons would not flow from cation to anion since the circuit would not be complete and a buildup of residue that collects from using the battery would render it useless as no charge would be created.
An important difference between modern batteries and classic galvanic cells is that new batteries are complete self-contained and require no salt bridges giving them an advantage over classic galvanic cells. Instead of salt bridges batteries use some form of an electrolyte, which simply put is a substance that can conduct electricity when dissolved in water. The flow of electrons in batteries is measured by the difference in electrical potential from the anode, oxidized part, and cathode, positive part, and is measured by a voltmeter. The voltage across these cells is called the batteries cell voltage, cell potential, and often referred to as the cell’s emf (electromotive force). It is important to remember that these are all measured in volts.
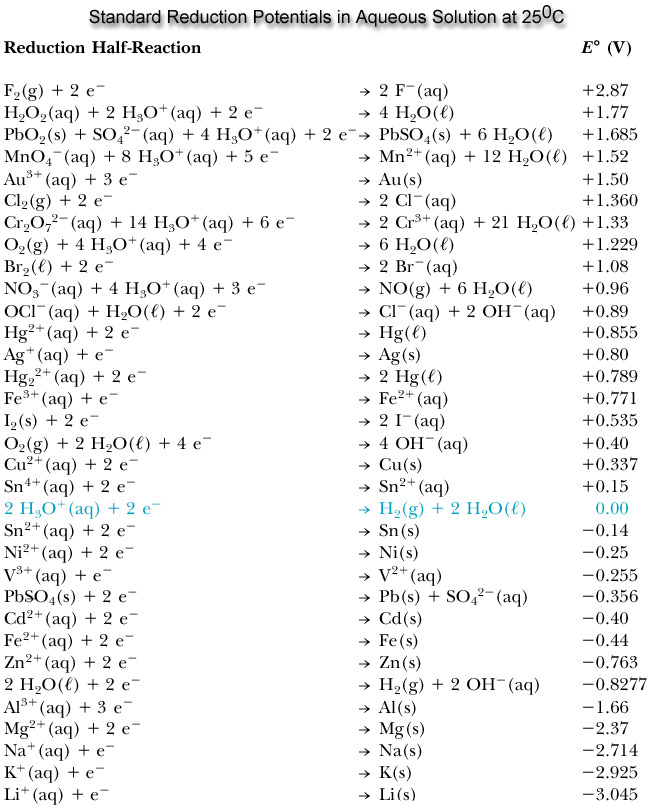
How does one determine what elements to use for cathodes and anodes though? Using standard reduction potentials one can see which elements are best for creating batteries. Looking at the equation for how cell potential is determined helps understand why certain elements are better as anodes and some as cathodes. Eºcell = Eºcathode-Eºanode remember the cathode is where reduction occurs and where electrons are gained. When looking at a standard table of reduction potentials it shows how likely an element is to be reduced or gain electrons thus the higher or more positive the reduction potential the more likely to be reduced and the lower or more negative the reduction potential the more likely the element is to be oxidized and lose electrons. This means that the best batteries are created by having an element with a high reduction potential to have a good cathode and an element with a very low reduction potential as the anode to create the biggest difference and highest voltage output.
Dry Cells
Dry cell batteries also known as the Leclanché cell are commonly used in devices such as flashlights. These batteries contain no fluid in them, hence the name dry cell. Dry cells have a graphite cathode rod in center and a zinc anode. These are both in contact with a mixture of manganese dioxide MnO2 and carbon which contact the outside of the rod and zinc plate to help with the flow of electrons. However, as mentioned earlier batteries need to have some form of electron carrier, whether it be a salt bridge or electrolyte solution of some form. In these cells a pasty like substance is used which contain electrolytes, usually composed of zinc (II) chloride ZnCl2 and ammonium chloride NH4Cl. This substance is used instead of a typical aqueous, dissolved in water, electrolyte because it creates a safer and less likely to leak battery. The battery then has a thin paper spacer around the paste, cathode, and anode and is then finally enclosed in a protective coating composed of some form of steel. Generally these cells produce around 1.5 volts of energy.

Lead Acid Storage Cells
Commonly seen in vehicles the lead storage battery is made up of six identical cells all joined together in series. Every one of the six cells is composed of a lead anode and a cathode made of lead dioxide PbO2 and both are on a metal plate which is in a sulfuric acid solution. The sulfuric acid acts as the electrolyte in this cell. If you look at your car battery these are enclosed in a plastic case. Lead storage cells output about 2.1 V per cell so in total the battery outputs around12.6 V of energy used for starting your vehicle and running the other electronic components including but not limited to your radio. The interesting and useful part of lead cell batteries is that they are able to be recharged. Recharging batteries means that you run the battery through the reverse process that it normally goes through by instead of the battery outputting energy, energy is inputted into the battery. This process is also called electrolysis.

Lithium-Ion Cells
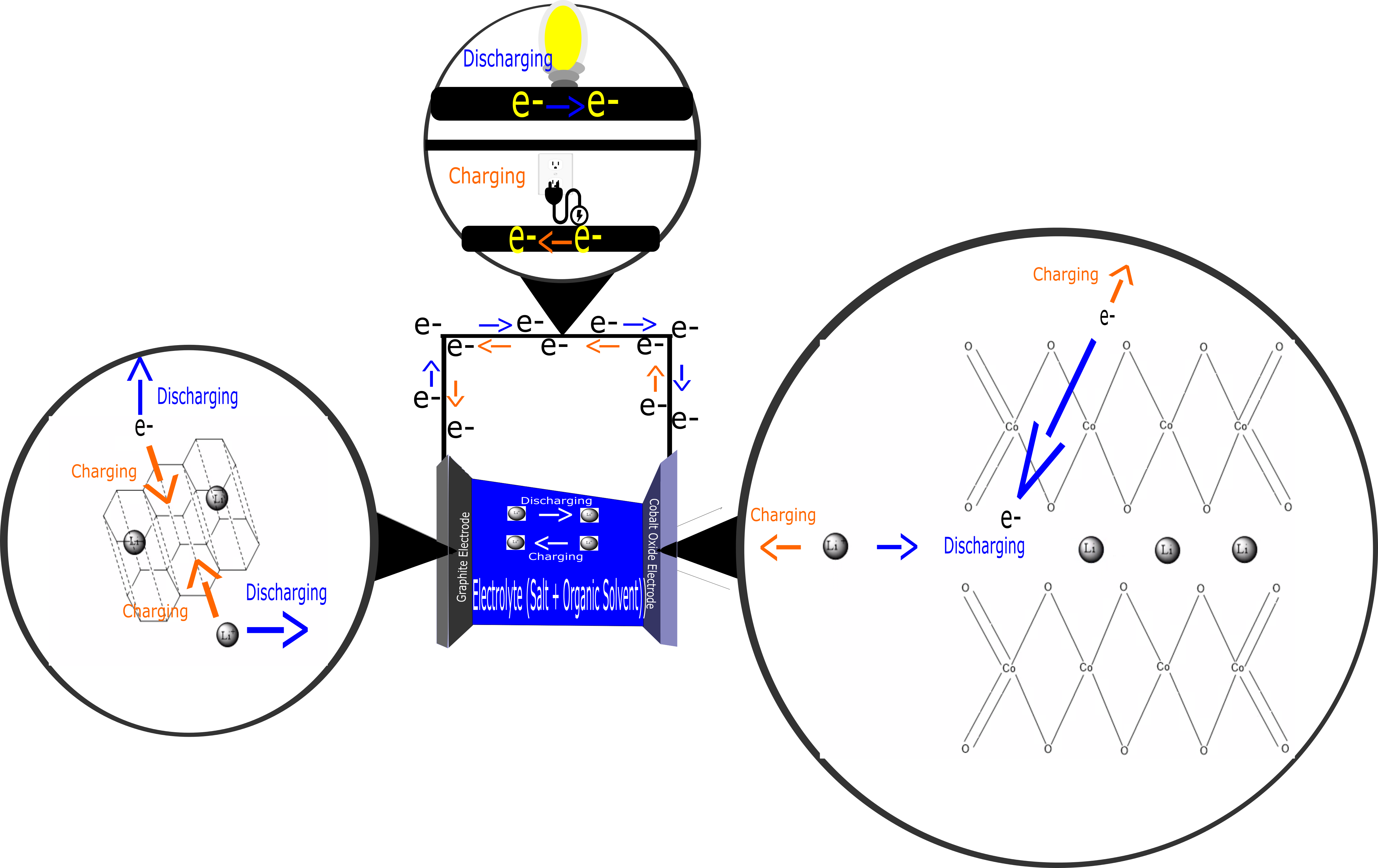
Lithium-ion batteries are known for powering everyone’s most valued devices in the twenty-first century; their phones, and computers. These are much smaller, lighter, and handle recharging extremely well making them very desirable for devices like laptops, cameras, cell phones, and other small frequently used electronics. Lithium-ion batteries are made up of a carbon based anode which is normally graphite and a transition metal such as cobalt oxide CoO2 for the cathode with a non-aqueous electrolyte in between the two. The non-aqueous electrolyte is composed of an organic solvent such as ethylene carbonate C3H4O3 and a dissolved salt which is often lithium perchlorate LiClO4 or lithium hexaflourophosphate LiPF6. These electrolytes are key to the functionality of the battery. Since the lithium salt is present a thin film or layer forms on the outside of the electrodes composed of compounds such as LiP or LiF this layer is called the solid electrolyte interphase or SEI layer. This deposit of ionic compounds causes the electrons to not continually flow through the battery. If electrons did it would essentially render the battery useless. However the SEI layer allows lithium ions to flow through and let the chemical redox reaction occur and electricity be produced. How cathodes and anodes are composed and work is very important in these batteries and the terms cathode and anode change when charging and discharging the battery. The carbonaceous anodes are porous and in these tiny spaces in the anode lithium Li and lithium ions Li+ are held. Since highly reactive transition metals are used in the cathode they can also hold Li+ which means that the ions and electrons can be held and transferred from anode to cathode creating energy. It should also be mentioned however that over time due to irreversible reactions the SEI layer will grow and begin to lower the capacitance of the battery. The main advantage to lithium-ion batteries is not their size and lightweight capabilities. These cells use lithium which is the lowest or most negative standard reduction potential giving it the best reducing strength and meaning that when combined with the proper cathode the maximum amount of voltage for a cell could be achieved. Normally these cells output around 3.4 V.